Aluminum-Copper HVAC Protection
The HVAC/Refrigeration industry is converting many components from copper to aluminum. The reasons? Aluminum is less expensive by weight, is more corrosion resistant, weighs less, and maintains similar heat-transfer rates to copper.
These aluminum parts are joined to the rest of the HVAC/R components via brazing. Aluminum-to-copper joints may be brazed with Lucas-Milhaupt AL 802 or AL 718 flux-cored Handy One® alloys. The flux in these brazing rods is non-corrosive and does not need to be removed from the joint after brazing.
rest of the HVAC/R components via brazing. Aluminum-to-copper joints may be brazed with Lucas-Milhaupt AL 802 or AL 718 flux-cored Handy One® alloys. The flux in these brazing rods is non-corrosive and does not need to be removed from the joint after brazing.
However, the damp environment in which HVAC/R units are used can cause an aluminum-to-copper joint to be susceptible to galvanic corrosion. Therefore, the joints must be protected. Let's explore this challenge and potential solutions.
Galvanic Corrosion
Every metal or conducting material has a different galvanic potential. If two metals with different potentials are placed in contact with one another in the presence of an electrolyte, current will flow between them. A return current will then flow, via the electrolyte, from the less noble to the more noble metal. In an HVAC/R environment, rain and condensation act as the electrolyte in the corrosion process, providing the connection to start the electron flow between the copper and aluminum tubes.
The less noble material becomes the anode, and the more noble material becomes the cathode. The less noble material sacrifices itself for the more noble material; in this case, the aluminum is sacrificed and the copper remains undamaged. When brazed joints involve dissimilar materials in direct contact, always consider the possibility of galvanic corrosion.
The corrosion speed is directly dependent upon the difference in potential between the two materials and the environment. According to the Aluminum Brazing Handbook: The rate at which a brazed aluminum joint, completely free of flux, will corrode in the presence of moisture is directly related to the solution potential difference that may exist between the alloys involved. The lower the potential difference, the lower the rate of corrosion. Potential differences of less than 0.013 volt are usually considered insignificant. An example of a Galvanic Chart for the electrolyte seawater is shown in Figure 1. The chart is used for illustrative purposes only.
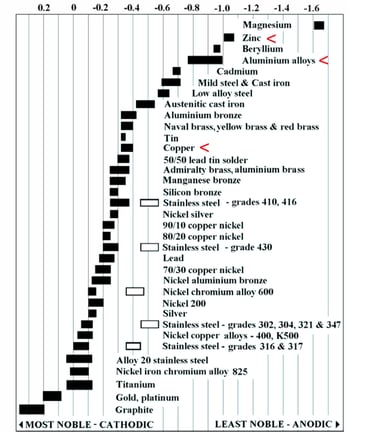
Figure 1: Galvanic Chart. The most noble materials (to the left) survive. The least noble materials (to the right) sacrifice. In HVAC/R, aluminum sacrifices (corrodes) so that the copper will survive (remain unaffected by corrosive environment). Zinc can sacrifice to save both aluminum and copper. Source: Atlas Steels, seawater-ambient temperature.
Protective Coatings
Manufacturers prevent galvanic corrosion by sealing aluminum-to-copper joints off from the environment. There are several products that work well, even though they are not made specifically for this industry. Manufacturers have "thought outside of the box" to find these solutions:
- ZRC® Cold-Galvanized Compound (zrcworldwide.com) - this is a spray-on or brushable zinc coating. Since zinc is not a very noble metal, it acts as the anode that sacrifices itself to save both the aluminum and the copper in a galvanic corrosion environment.
- Permatex® Spray Sealant Leak Repair (permatex.com) - this product is designed to stop leaks in car engines, but can also be used to seal copper-to-aluminum joints from the environment. When sprayed over the surface of the joint interface, it forms an air-tight rubber seal over the brazed joint.
- 3MTM Heat Shrink Tubing EPS-300 (3M.com) - this tubing is designed to protect electrical components and wire bundles from the environment. It is a rubber tube that shrinks when heated. Once the tube shrinks against the surface of a copper-to-aluminum joint, it secretes a melted adhesive that further protects the surface from corrosion.
CONCLUSION:
The HVAC/R industry is converting many components from copper to aluminum. These aluminum parts are typically joined to copper refrigeration components, and may be brazed with Lucas-Milhaupt AL 802 or AL 718 flux-cored alloys. However, the joints must be protected from galvanic corrosion in the HVAC/R environment, with solutions including ZRC zinc coating, a Permatex air-tight rubber seal, or 3M heat-shrink tubing.
Thanks for joining us today! Lucas-Milhaupt is dedicated to providing expert information for Better Brazing; please feel free to share this blog posting with associates. For a demonstration of joining aluminum to copper, see our video. As always, contact Lucas-Milhaupt whenever we may be of assistance.

