Flux Defined
Flux, according to the American Welding Society (AWS), is "a material used to hinder or prevent the formation of oxides and other undesirable substances in molten metal and on solid-metal surfaces, and to dissolve or otherwise facilitate the removal of such substances."
In other words, flux is a chemical compound, usually made in the form of a paste, slurry, liquid, or powder, which is applied to braze joint surfaces just before brazing. As the temperature of the joint area is increased, the flux melts and becomes active. Once active, the flux has three primary functions:
- Absorb and dissolve residual oxides on the base metal surface and filler metal
- Prevent the oxidation of metal surfaces during the heating operation by excluding oxygen
- Assist in the flow of the alloy by presenting a freshly cleaned surface for the molten alloy
Silver Brazing Fluxes
Fluxes for silver brazing are typically composed of potassium salts or fluorides and borates in a water base. Several flux types are available to cover all possible base metals and/or conditions during brazing. Therefore, the base metal, filler metal, and brazing process/heating method must be examined to select the best flux for the job.
AWS has developed the specification A5.31 to cover the classification of fluxes. Included in this specification are classifications of fluxes used for silver-based filler metals. This specification also recommends the various base metals, filler metal combinations, and temperature ranges in which the various fluxes are effective (see Figure 1). Additional information on fluxes can be found in the Brazing Handbook, 5th Edition, published by AWS.
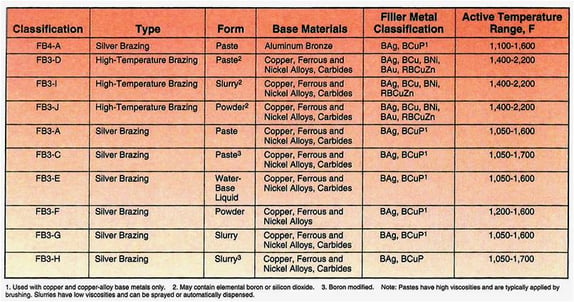
Figure 1. Specifications for Fluxes
Base Metals
Base metals vary in their rate of oxide formation and in the character of their oxides. Fortunately, it is possible to formulate general-purpose fluxes that can accommodate the oxides of most metals used in brazed assemblies.
Silver, copper, and brass form light oxides that are easily removed. Because of their higher thermal conductivity, the danger of localized overheating is lessened. By using low-temperature silver-alloy filler metals, the rate of oxide formation is minimized. The flux typically used is Lucas-Milhaupt's Handy Flux®, which is classified by AWS as a FB3-A flux. In some cases, the flux may be diluted with water to reduce the concentration of flux.
Bronzes containing aluminum or other base metals with small percentages of beryllium, silicon, or titanium may require the use of Handy Flux Type A-1 (FB4-A) to remove their stable, tenacious oxides.
Carbon and low-alloy steels typically oxidize more rapidly than the nonferrous materials. The general fluoride/borate-type flux, such as Handy Flux (FB3-A), will dissolve the oxides, but the consistency of the flux should be used in standard form, not diluted. With some larger parts, Handy Flux Type B-1 (FB3-C) may be more appropriate because of its extended heat range/life. The extended heat range and/or life of the Handy Flux Type B-1 is a result of the addition of elemental boron. The boron also improves the fluxing of refractory oxides that form from the elements chromium and tungsten. These elements are common in stainless steels, nickel alloys, and carbides, which will benefit from a flux such as Handy Flux Type B-1.
Filler Metals
Fluxes must be chosen to be effective during the melting and flow of the filler metals to be used. The solidus (melt) and liquidus (flow) temperatures of the filler metals must be reviewed. Ideally, the flux will melt and be active below the solidus temperature of the filler metal, and it will also be active above the liquidus range of the filler metal chosen.
Two fluxes that have different active temperature ranges are the Handy Flux (FB3-A), active from 1,050-1,600°F (566-871°C), and Handy Flux Hi-Temp (FB3-D), active from 1,400-2,200°F (760-1,204°C). As an example, steel can be brazed to copper using a filler metal with a liquidus temperature of 1,205°F (652°C). With this combination of base metals and filler metal, the correct flux to use would be type Handy Flux (FB3-A). The filler metal melts within the active range of the recommended flux.
These same base metals could also be joined with a higher-temperature filler metal. If the liquidus temperature of this filler metal was 1,575°F (857°C), an alternate flux would be more appropriate than the Handy Flux (FB3-A). In this situation, Handy Flux Hi-Temp (FB3-D), which is active from 1,400-2,200°F (760-1,204°C), should be used.
Brazing Process/Heating Method
Time and temperature influence oxide formation. If higher temperatures and longer heating times are encountered when brazing, fluxes of the Handy Flux Type B-1 (FB3-C) or Handy Flux Hi-Temp (FB3-D) type may be required. However, if heating times and temperatures are low, diluted fluxes, slurries, or liquid-type fluxes may be used.
In accordance with the AWS classifications, special fluxes have been developed in which the tendency for the flux to boil off a part has been minimized. This situation typically arises in a fast-heat process, such as induction brazing, or in processes where small parts are brazed.
Automation also plays an important role in choosing a flux. The ability of a flux to be automatically deposited at a joint is becoming more important in manufacturing. Fluxes must accommodate being sprayed or deposited without separation. For more information, read about Handy-Flo Dispensable Fluxes.
Conclusion
For silver brazing, several flux types are available for various base metals and brazing conditions. Consider your base metal, filler metal, and brazing process/heating method; then choose the correct flux.
Questions? Lucas-Milhaupt's experts can help you navigate the challenges of joining metals. For further information on process, applications, and flux types, please visit our Handy Flux page.

