What is dezincification?
First, we must understand dealloying-a type of corrosion involving selective leaching or removal of one element or phase with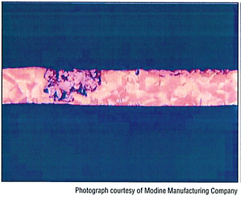 in the material. This selectively removed phase or element from an alloy, leaves behind a porous, spongy mass.
in the material. This selectively removed phase or element from an alloy, leaves behind a porous, spongy mass.
Dezincification is a form of dealloying corrosion that involves a preferential leaching of zinc (from brass). The corrosion continues below the surface, with weak, porous copper replacing the brass. Eventually, dealloying will penetrate the metal, allowing leaks throughout the structure.
Photo: Dezincification in brass. Courtesy AWS Brazing Handbook, 5th edition.
There are two types of dezincification: plug-type or uniform.
- Plug-type dezincification is localized, minimally or not affecting the surrounding area. Watch for plug-type dezincification when encountering neutral or alkaline water, with a high salt content, above room temperature.
- Uniform dezincification means that the element's active component leaches out over the surface. Watch for this condition when you are working with slightly acidic water, with a low salt content, at room temperature.
Conditions for dezincification
You will see more dezincification in materials with a zinc content of 15% or more. Acidic aqueous environments and high-electrode potentials will cause dezincification of these materials. Selective dezincification is more likely in hot, polluted, unaerated water under stagnant conditions. Zinc-bearing materials are also more susceptible to selective attack if they contain permeable layers of corrosion products, deposits or crevices.
Dezincification as related to Braze Filler Metals
Silver braze filler metals can sometimes be thought of as very similar to brass base metals. That in mind silver braze filler metals that are composed of silver-copper and zinc are subject to dezincification. We have observed dezincification when alloys such as Easy-Flo® 3 or other zinc-bearing silver brazing alloys are used on copper-nickel tubing exposed to saltwater. In these cases, adding nickel to zinc-content brazing alloys is not helpful. In this example the Easy-flo 3 exhibited the dezincification and leaked in service through the joint.
Influencing factors
Dezincification is influenced by the composition differences between base metals and the braze filler metals used to joint them. RBCuZn alloys can contain zinc in excess of 40%. When used on base materials such as copper-nickel which are entirely absent of zinc, the assembly will be highly susceptible to dezincification in corrosive environments.
Remember, perfect conditions for attack involve hot, polluted, stagnant water. Thick layers of corrosion products, deposits or crevices that are permeable will also invite dezincification.
Solutions to dezincification
Adding (corrosion-resistant) nickel to zinc-bearing silver filler metal is not sufficient to prohibit dezincification in highly corrosive conditions such as seawater. For example, Lucas-Milhaupt's Silvaloy® 505, which contains 2% nickel, would be of little help when you are using copper-nickel tubing and exposing a component to saltwater corrosion. Instead, use a zinc-free filler metal such as Silvaloy 603 to avoid dezincification under corrosive conditions.
Silvaloy® 603, the AgCuSn alloy, is one of the best brazing alloys for copper-nickel tubing that will be exposed to heated salt water. Fabricators of seawater converters have used this product for many years with success. Keep this alloy in mind for seawater converters involving  assemblies of 90-10 or 70-30 copper-nickel tubing.
assemblies of 90-10 or 70-30 copper-nickel tubing.
For standard copper tubing used in fresh water or seawater applications at room temperature, customers have been using Sil-Fos® successfully for more than 25 years.
In addition, these guidelines can help you avoid dezincification in brazing assemblies:
- Avoid submerging zinc-bearing materials in corrosive environments such as salt water or low-pH situations.
- Avoid bonding CuNi, CuSi and CuSn base materials with zinc-bearing filler materials such as RBCuZn, or low-temperature filler materials in the Silvaloy family which also contain zinc.
Conclusion:
Dezincification is a form of dealloying corrosion that involves a preferential leaching of zinc (from brass or zinc bearing braze filler metals). Dezincification is influenced by the composition differences between base metals and the braze filler metals used to join them. Solutions include using zinc-free brazing materials, avoiding submerging zinc-bearing materials in corrosive environments and avoiding bonding affected base materials with high zinc-bearing filler materials.
Questions? Contact us for further assistance. For detailed questions regarding specific applications, please call Lucas Milhaupt's Technical Department at 800.558.3856.
We are pleased to providing expert information for Global Brazing Solutions®. Feel free to share this posting with associates, and save our blog site to your Favorites for easy reference!

