Brazing of Beryllium-Copper Alloys
Beryllium copper offers high corrosion resistance, electrical conductivity and thermal conductivity, plus high strength and resistance to high temperatures. Non-sparking and non-magnetic, it is useful in mining and petrochemical industries. With high resistance to fatigue, beryllium copper is also used for springs, connectors and other parts subject to cyclical loading.
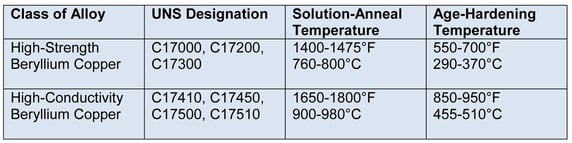
Brazing beryllium copper is relatively inexpensive and easily performed without weakening the alloy. Beryllium-copper alloys are available in two classes: high-strength C17000, C17200 and C17300; and high-conductivity C17410, C17450, C17500 and C17510. Thermal treatment further strengthens these alloys.
Metallurgy
Brazing temperatures for beryllium-copper alloys are typically above the age-hardening temperature and approximately the same as the solution-annealing temperature.
General steps for heat treating beryllium-copper alloys follow:
First, the alloy must be solution annealed. This is accomplished by dissolving the alloy into a solid solution so it will be available for the age-hardening step. After solution annealing, the alloy is quickly cooled to room temperature by water quenching or using forced air for thin parts.
The next step is age hardening, whereby sub-microscopic, hard, beryllium-rich particles are formed in the metal matrix. Aging time and temperature determine the amount and distribution of these particles within the matrix. The result is increased strength of the alloy.
Alloy Classes
1. High-strength beryllium copper - Beryllium copper is normally purchased in the solution-annealed condition. This anneal consists of heating to 1400-1475°F (760-800°C), followed by a quick quench. Brazing can be accomplished either in the solution-annealing temperature range-followed by a quench-or by very rapid heating below this range, without affecting the solution-annealed condition. The temper is then produced by aging at 550-700°F (290-370°C) for two-to-three hours. With other beryllium alloys containing cobalt or nickel, heat treatment may vary.
2. High-conductivity beryllium copper - The composition predominantly used in industry is 1.9% beryllium-balance copper. However, it can be supplied with less than 1% beryllium. Where possible, the lesser-beryllium-content alloy should be employed for the best brazing results. Anneal by heating to 1650-1800°F (900-980°C), followed by a quick quench. The temper is then produced by aging at 850-950°F (455-510°C) for one to eight hours.
Table 1. Properties of Beryllium-Copper Alloys
Cleaning
Cleanliness is vital for successful brazing. Pre-cleaning the braze-faying surfaces to remove oils and grease is essential to good joining practice. Note that cleaning methods should be chosen based on oil or grease chemistry; not all cleaning methods are similarly effective in removing all oils and/or grease contaminates. Identify the surface contaminant, and contact the manufacturer for the proper cleaning methods. Abrasive brushing or acid pickling will remove oxidation products.
After cleaning components, braze immediately with flux to provide protection. If components must be stored, parts may be protected with electroplate of gold, silver or nickel to 0.0005" (0.013 mm). Plating can be used to facilitate the wetting of the beryllium-copper surface by the filler metal. Both copper and silver may be plated 0.0005-0.001" (0.013-0.025mm) to hide the difficult-to-wet oxides formed by beryllium copper. After brazing, remove flux residues with hot water or mechanical brushing to avoid corrosion.
Design Consideration
Joint clearances should allow flux to escape and also provide sufficient capillarity, depending on the filler-metal chemistry chosen. Uniform clearances should be 0.0015-0.005" (0.04-0.127mm). To assist in displacing flux from joints-especially those joint designs which utilize preplaced strip or strip preforms-movement of one faying surface with respect to the other and/or vibration may be employed. Remember to calculate clearances for the joint design based on the anticipated brazing temperature. In addition, beryllium copper's expansion coefficient is 9.7 x 10-6/°F (17.0 x 10-6/°C). Consider thermally induced strains when joining metals with different thermal-expansion properties.
Brazing Procedures
Flux is generally required with all heating methods for brazing beryllium-copper alloys; Lucas-Milhaupt's Handy Flux or Ultra flux may be employed with all methods. If required, fluxes such as Handy Flux Type B-1, UltraFlux Black, and Handy Flux Type A-1 may be used, if the size or chemistry of one brazing surface requires a special flux.
Brazing high-strength beryllium copper - Small parts may be torch, resistance or induction brazed in the solution-annealed condition with a low-temperature alloy, melting and flowing between the aging and solution-anneal temperature, then rapidly air cooled or quenched, followed by the aging procedure. This is possible because the brazing operation does not affect the solution-annealed condition to any great degree. These heating methods are best if the heat is concentrated close to the joint and the brazing is accomplished in as fast a cycle as possible. Resistance (usually an adapted spot welder) should be considered where possible.
Alloys used to braze beryllium copper in the solution-annealed condition are Lucas-Milhaupt Easy-Flo 45, Easy-Flo, Braze 560, Braze 501 and Braze 600, but in the case of brazing beryllium copper to steel, Easy-Flo 3 and Trimet 258 (Easy-Flo 3 Trimetal) give stronger joints. When joining beryllium copper to steel, the beryllium will contaminate the silver-brazing alloy and cause dewetting of the steel. This is overcome by using the Trimetal. Copper in the Trimetal acts as a barrier, preventing the beryllium from contaminating the brazing alloy in contact with the steel.
Lucas-Milhaupt Sil-Fos may be used for brazing beryllium copper. However, as with the other silver-brazing alloys, flux is necessary. Even with flux, joints do not have the strength of those made with Easy-Flo 45; the Sil-Fos joints appear relatively porous. Sil-Fos has been used without flux, but silver plating of the beryllium copper is necessary if this method is employed.
High-strength beryllium copper can also be brazed and solution heat treated at the same time. Large parts, dissimilar sections or long-cycle work may be brazed in a furnace with an alloy (Braze 720/721) flowing at 1435°F (779°C). Even in most inert or reducing atmospheres, brazing flux will be required. This brazing should be accomplished at 1450-1500°F (790-816°C). Then, the parts should be quenched from a temperature just below 1435°F (779°C). The final step will be aging.
Brazing high-conductivity beryllium copper - Similar to the high-strength beryllium copper, small parts may be torch, resistance or induction brazed in the aged condition with a low-temperature alloy, melting and flowing between the aging and solution-anneal temperature, then rapidly air cooled or quenched. These heating methods are best if the heat is concentrated close to the joint and the brazing is accomplished in as fast a cycle as possible. Resistance (usually an adapted spot welder) should be considered where possible.
Alloys used to braze beryllium copper in the aged condition are Lucas-Milhaupt Easy-Flo 45, Easy-Flo and Braze 560. Mechanical properties will be affected, depending on the time and the temperature to which the beryllium-copper base material is exposed. Run samples and evaluate mechanical properties to validate the manufacturing process. 
High-conductivity beryllium copper can also be brazed and solution-heat treated at the same time. When high-temperature brazing high-conductivity beryllium-copper alloys, use filler metals flowing at or slightly above the 1660-1740°F (900-950°C) range. Filler metals such as AWS RBCuZn-D (C77300), Braze 559 and Premabraze 130/131, with a melting temperature above the solution-anneal temperature of the base material, are successfully brazed and heat treated simultaneously.
Safety
There are typically few health issues associated with handling beryllium copper in solid form, but safe handling practices are advised to avoid health risks. Inhalation of beryllium-copper dust may cause serious lung disorders; consult OSHA guidelines for occupational respiratory exposure.
CONCLUSION:
Beryllium copper, when fully heat treated and cold worked, is the hardest and strongest of copper alloys. Alloys are available in two classes: high strength and high conductivity. Brazing beryllium copper is relatively inexpensive and easily performed without weakening of the alloy. Beryllium copper offers high corrosion resistance, electrical conductivity and thermal conductivity, plus high strength and resistance to high temperatures.
Lucas-Milhaupt provides a complete line of braze alloys for your manufacturing operation. Please contact us for assistance if you have questions regarding use of beryllium-copper alloys.

