Interfacial Corrosion
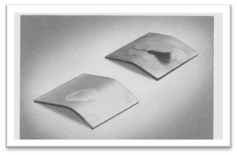
The situation: When brazing stainless steel with flux, subject to a damp environment, you notice rust-like spots around the periphery of the braze metal. Rapid corrosion may begin within two days or even as soon as two hours. The brazing filler metal may eventually peel away from the stainless steel surface as illustrated in the bent samples of Figure 1.
 |
|
Figure 1: Bent after brazing (left); bent displaying interfacial corrosion after exposure to tap water (right). |
Ultimately, complete separation of the filler metal from the stainless steel may occur, resulting in complete failure.
Why does this happen? How can the problem be avoided? Join us for a discussion on the science behind this issue and the solutions that help avoid interfacial corrosion.
Interfacial Corrosion Defined
Interfacial corrosion (sometimes called knife-line attack) is a phenomenon involving corrosion along the interface between the brazing filler metal and the base metal. A brazed joint may be susceptible to interfacial corrosion depending on a number of factors, including: the types of base metal (stainless steel) and brazing filler metal used, the brazing technique (using flux), and the service environment (exposure to moisture). One very common occurrence involves the brazing of stainless steel using a flux and silver based braze filler metal.
Theory behind Interfacial Corrosion
Why does interfacial corrosion occur when brazing stainless steel? One very common corrosion failure occurs when flux is introduced. During brazing, flux removes the surface oxide layer on the base metal at the brazed joint in order for bonding to take place. In metals that depend on a passified layer (created by Ni or Cr) for corrosion resistance, this area (interface) at the brazed joint now has less corrosion resistance. During service, this area of reduced corrosion resistance is more susceptible to attack at the interface.
To explain more fully, let's take a quick look at the brazing process. The flux is applied and the stainless steel is heated. Chromium oxide is removed from the surface by the molten flux. After brazing, a thin layer of chromium-free iron may be left exposed at the surface of the stainless steel. When the iron-rich surface is exposed to water or dampness-especially with the presence of a brazing filler metal to which the iron would be anodic-the free iron turns into rust on the surface of the stainless steel, just outside of the brazed area. This corrosion at the fillet edge can additionally form a crevice. Once the crevice defect is formed further corrosion takes place at the interface between the filler metal and stainless steel. Corrosion can continue until complete separation of the filler metal from the stainless steel occurs.
Prevention with Nickel
When using 300 series stainless steels, a brazing filler metal containing a small amount of nickel can eliminate interface corrosion completely and cost effectively. The nickel-iron surface created is more resistant to corrosion than an iron surface, even in the presence of moisture and chlorides. Lucas-Milhaupt Braze 495, Braze 505, and Easy-Flo 3 contain a small percentage of nickel which provides the desired nickel-rich interfacial layer next to the steel for a successful fillet that can resist corrosion.
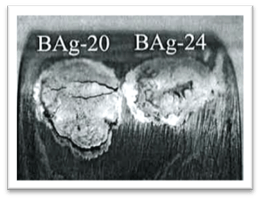
Figure 2 shows both a non-nickel brazing filler metal and a nickel-bearing silver filler metal brazed onto a 304 series coupon, then exposed to water for 20 days.
 |
|
Figure 2: Non-nickel- vs. nickel- bearing filler metals brazed, then exposed to water. |
Separation of the non-nickel-bearing filler metal (BAg-20) is observed. No separation is noted on the nickel-bearing filler metal (BAg-24) sample.
Please note: Brazing filler metals that work with 300 series stainless steel may not supply enough nickel to the fillet edge to keep 400 series stainless steel surfaces nickel rich. Interfacial corrosion may be retarded, but not eliminated. Therefore, the fillet edges could leave joints vulnerable to corrosion.
For 400 series stainless steel, we recommend the use of Lucas-Milhaupt Braze 630 product. This alloy has been found to be practically immune to interfacial corrosion. It does require a higher brazing temperature, and operators will note that it flows in a rather sluggish manner. This slower flow is partly responsible for the improved corrosion performance. It does not form thin fillet edges, and hence, the desired nickel-rich layer extends all the way to the fillet edge to protect against corrosion.
Conclusion
When brazing series 300 or 400 stainless steels with flux, you can avoid interfacial corrosion by using alloys containing nickel.
Lucas-Milhaupt braze products with nickel include Braze 495, Braze 505, Easy-Flo 3, and Braze 630. These products will help you to braze assemblies with strong, successful fillets for reliable performance in the field.
For more information on better brazing products or services, or if we can be of further assistance to you, please contact Lucas-Milhaupt.

